Our research interests include:
- Renewable energy: Optical spectroscopy of pigment-protein light-harvesting photosynthetic complexes - energy transfer and charge transfer;
- Biophotonics / protein structure and [low-temperature] dynamics: Protein-chlorophyll complexes as model systems. Heat transfer in proteins and through protein-water interfaces. Energy landscapes and dynamics in amorphous solids;
- Biosensors for explosives detection utilizing photosynthetic reaction centers;
- Combining optical (Raman) and transport measurements in graphene and carbon nanotubes.


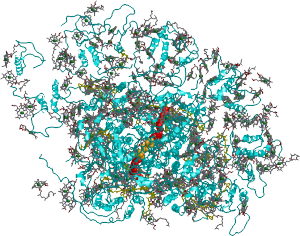 Top view of plant Photosystem I. Blue: protein. Grey: chlorophylls. Yellow: carotenoids. Red: quinones, Orange: FeS clusters.
Top view of plant Photosystem I. Blue: protein. Grey: chlorophylls. Yellow: carotenoids. Red: quinones, Orange: FeS clusters.
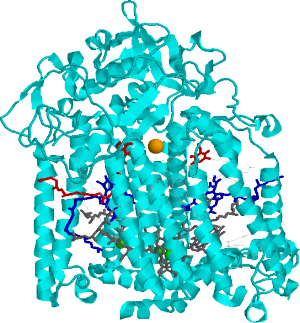 Side view of the bacterial reaction center (BRC). Grey: bacteriochlorophylls. Dark-blue: bacteriopheophytines, Red: quinones, Orange: non-heme iron.
Side view of the bacterial reaction center (BRC). Grey: bacteriochlorophylls. Dark-blue: bacteriopheophytines, Red: quinones, Orange: non-heme iron. 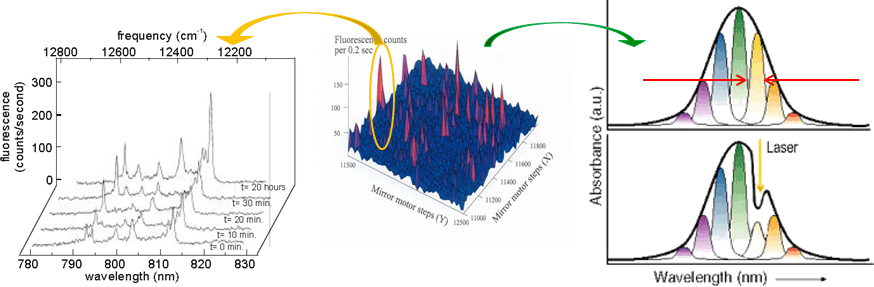 Relationship between single photosynthetic complex spectroscopy and spectral hole burning. The raster-scan image of the sample containing multiple complexes (of the same type, e.g. LH2) is in the center. One can select a single complex (left, one complex contains multiple chlorophyll molecules, hence multiple spectral lines, © Köhler’s Group, Bayreuth), or one can work with the whole ensemble (right) and select molecules in resonance with the narrow-band laser. Then a small conformational change of the environment may be triggered by light, shifting the absorption of these molecules.
Relationship between single photosynthetic complex spectroscopy and spectral hole burning. The raster-scan image of the sample containing multiple complexes (of the same type, e.g. LH2) is in the center. One can select a single complex (left, one complex contains multiple chlorophyll molecules, hence multiple spectral lines, © Köhler’s Group, Bayreuth), or one can work with the whole ensemble (right) and select molecules in resonance with the narrow-band laser. Then a small conformational change of the environment may be triggered by light, shifting the absorption of these molecules.