Under certain acoustic conditions, these microbubbles can also be made to induce bioeffects, including the local and reversible opening cell membranes to allow entry of therapeutics – sonoporation. Either through co-injection of a therapeutic or through attaching the payload to the bubble itself, microbubbles are emerging as a targeted drug/gene delivery vehicle – for example in cancer and cardiovascular disease applications – whereby drug deposition is limited to the precise location where the microbubbles intersect with the focused ultrasound beam.
We are an interdisciplinary research group at the interface of physics and biology. Our research projects focus on both the acoustics and biophysics of biomedical ultrasound therapeutics.
Our first research interest is in studying the basic physics of ultrasound-stimulated microbubble vibrations, and other acoustically sensitive agents. A more complete understanding of the dynamic response of bubbles will aid in contrast image quality and quantification, optimal microbubble design for a given application, and controlled and repeatable bioeffects.
A second research theme is to understand the cellular and vascular biophysics of ultrasound-microbubble therapies. We are interested in learning about the key signaling molecules that initiate, sustain and reverse sonoporation, and how those are linked to the physical acoustics of bubble vibrations.
A third research theme is to develop a dual imaging and therapeutic targeted drug/gene delivery ultrasound platform. We will combine aspects of cell/vascular biology, ultrasound acoustic physics and medical imaging to develop an image-guided approach to local and safe drug/gene delivery in models of cardiovascular disease.



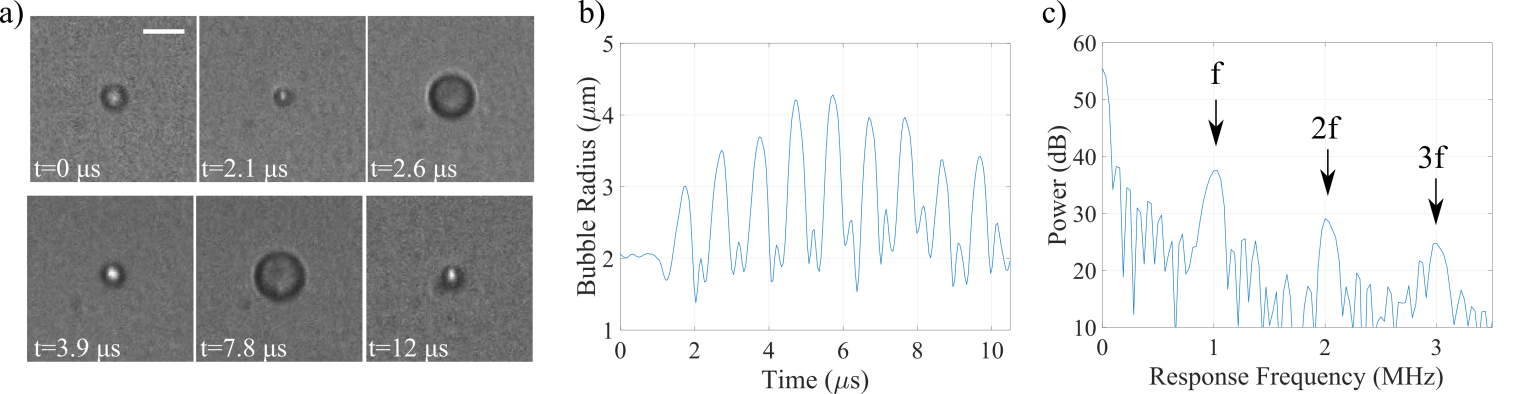 An oscillating microbubble under an ultrasound field (frequency of 1 MHz, peak-negative pressure of 400 kPa, pulse duration of 8 µs) captured at 10.86 million fps. Scale bar is 5 µm. Adapted from Helfield et al. Ultrasound in Medicine and Biology 42(3) 2016. b) Radial cavitation dynamics and c) associated power spectrum. Under such an acoustic regime, this bubble oscillates at the fundamental (f), second (2f) and third (3f) harmonic frequencies, exhibiting distinctly nonlinear behaviour.
An oscillating microbubble under an ultrasound field (frequency of 1 MHz, peak-negative pressure of 400 kPa, pulse duration of 8 µs) captured at 10.86 million fps. Scale bar is 5 µm. Adapted from Helfield et al. Ultrasound in Medicine and Biology 42(3) 2016. b) Radial cavitation dynamics and c) associated power spectrum. Under such an acoustic regime, this bubble oscillates at the fundamental (f), second (2f) and third (3f) harmonic frequencies, exhibiting distinctly nonlinear behaviour.
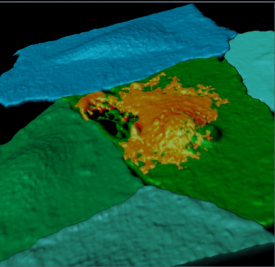 Pictured is a fluorescently labeled endothelial cell monolayer, pseudocolorized in blue/green, and imaged using confocal microscopy. One cell has been selectively perforated via ultrasound-induced microbubble vibration allowing the entrance of a model therapeutic (orange). This perforation re-seals after 30 minutes. See Helfield et al. PNAS 113(36) 2016.
Pictured is a fluorescently labeled endothelial cell monolayer, pseudocolorized in blue/green, and imaged using confocal microscopy. One cell has been selectively perforated via ultrasound-induced microbubble vibration allowing the entrance of a model therapeutic (orange). This perforation re-seals after 30 minutes. See Helfield et al. PNAS 113(36) 2016.





