Keroles Riad is a doctoral candidate in the Individualized program (INDI). Keroles holds a BEng in Mechanical engineering and an MSc through the INDI program. Keroles is a recipient of the Quebec Lieutenant Governor Youth Medal, and is the driving force behind Concordia’s successful Waste Not, Want Not student-led composting program.
Blog post
I set things on fire intentionally (most of the time)
 The next best thing to playing with fire is playing with electricity. Credit: Dainius Juras.
The next best thing to playing with fire is playing with electricity. Credit: Dainius Juras.
I develop new materials for stereolithography 3D printing that are stable under sunlight. Normally, the 3D printing resin that is used in this technology consists of epoxy (glue) and an initiator. The initiator triggers a reaction that solidifies that glue upon exposure to the right kind of light (UV). For reference, the glue you buy from the dollar store solidifies as it is exposed to heat. My glue solidifies as it is exposed to light. The stability issue I am trying to address originates from the fact that the same UV that is in the 3D printing laser is also present in sunlight (why you put sunscreen at the beach… remember?). So, I need initiators that respond exclusively to light that is outside the solar spectrum on Earth. If successful, engineers can take full advantage of this 3D printing technology which is currently limited to modeling due to those instability issues.
I plan to achieve my goal by using an exceptionally small kind of nanoparticles called quantum dots as initiators. The superpower of quantum dots is that the light they respond to depends on their size. That means that if quantum dots can solidify glue upon exposure to light, then I can change the light they respond to by controlling their size, and hopefully have a stable 3D printing resin. Nanoparticles are particles whose size is in the order of nanometers. A nanometer is 40,000 times smaller than the thickness of one human hair. Quantum dots are limited to the extreme end of that scale (smaller than 10 nm). I have demonstrated that semiconducting nanoparticles can initiate epoxy solidification reaction upon exposure to UV light which has recently been published here.
The next step is to make the quantum dots that respond to the right kind of light. Thus, I wanted to learn about how to make nanoparticles, and that is when things started getting on fire figuratively and literally, intentionally and unintentionally. I initiated a collaboration with the Particles Technology Laboratory (PTL) in ETH Zurich where I spent 9 months over two research internships. I am particularly grateful to the Concordia Institute of Aerospace Design and Innovation (CIADI), for supporting the first internship. PTL, led by Professor Sotiris Pratsinis, is the world-leading research laboratory on the Flame Spray Pyrolysis (FSP) technology that can produce almost any metal oxide nanoparticles and composites at an industrial scale. Essentially, you mix chemicals that contain the desired metal elements in flammable solvents and you literally put the whole thing on fire (It is extremely satisfying!), by spraying the mixture through an open flame. Everything gets combusted and oxidized: Hydrogen becomes H2O, Carbon becomes CO2, and metals such as Titanium become metal oxides (TiO2). The metal oxide particulates collide in the flame and grow into nano or micro-particles. The particles are then separated from the aerosol phase by a filter on top of the flame where I would collect the particles I made. The time the particulates spend at the hot portion of the flame controls particle properties such as crystal size and surface area.
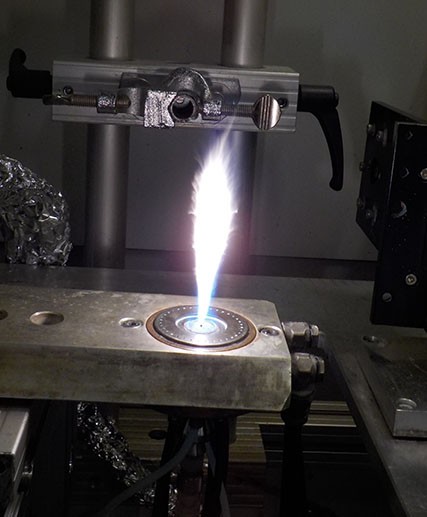 The FSP's open flame.
The FSP's open flame.
 PTL group picture at the end of my first internship.
PTL group picture at the end of my first internship.
Following my internships, a few things happened:
First, I did indeed manage to make the quantum dots the I wanted- soon to become my first open access paper. Sidenote: I refer you to my earlier rant at The Conversation about the inability of taxpayers to access the research they pay for because it is behind paywalls. In the process, I also stumbled into discovering a crystal structure that was never observed before in flame-made titania, published here. Second, I convinced my professor, Paula Wood-Adams, to let me write a major grant in collaboration with Professors Ali Dolatabadi and Christian Moreau, to buy the first FSP in Canada (now housed in the Hall building).
 Concordia's FSP- the first in Canada
Concordia's FSP- the first in Canada
Finally, international conferences became the best way to keep regular contact with my Swiss collaborators. Not surprisingly, that thought has occurred to the many alumni of PTL, and so I got to meet so many of the people whose research papers I have been reading and learning from. In 2018, I went to the World Congress of Particle Technology in Florida where I met Professor Alexandra Teleki from Uppsala University, Sweden. This year, I attended the 4th international symposium on gas phase synthesis where I virtually met Professor Reza Kholghy, from Carleton University here in Canada and who will be acquiring the second FSP in Canada. And I want you to meet them!
On March 24, I will be moderating a free virtual webinar hosted by the 4th SPACE with Professors Pratsinis, Teleki and Kholghy, which you can (and should!) register for here. The discussion will revolve around how nanotechnology is affecting your wine, gut and climate. Professor Pratsinis from ETH, Switzerland, is the world-leading authority on the FSP technology and will talk about gas-sensing applications which including diagnosis of diseases such as diabetes, and measuring if your wine has the right alcohol percentage. Did you know that SAQ’s online sales increased by 200% during the pandemic? Would not you want to know you are getting your money’s worth? Professor Teleki from Uppsala University, Sweden, researches pharmaceutical nanotechnology applications. And will talk about how flame-made magnetic nanoparticles: your local guide in the gut. Professor Kholghy from Carleton University, Canada, uses the FSP as a screening tool for renewable jet fuels to mitigate harmful impacts on climate.
 From left to right: Professors Sotiris Pratsinis, Alexandra Teleki and Reza Kholghy.
From left to right: Professors Sotiris Pratsinis, Alexandra Teleki and Reza Kholghy.
COVID has brought public health to the forefront of societal discourse. It has also brought to focus the tremendous impact technology has on our lives from remote work to virtual events (like this webinar) to 3D printing used to manufacture critical personal protective equipment (read my Op-Ed in the Montreal Gazette), to the record pace we have developed not one but four vaccines so far. Further, Montreal is the third-largest aerospace hub in the world. During the last climate strike, the largest protest in history, Montreal had the largest turnout of any city anywhere in the world. Technology, health, and climate are interconnected in so many ways. I am confident we will have a very interesting discussion that you will enjoy, and I am eager to learn alongside you.
PS: I do not have a good picture of me playing with fire, so I figured electricity must be the next best thing 🤷.
About the author


