PUBLICATIONS
Full publication list
Jaunky, D.B.; Laroque, K.; Liu, J.T.; Forgione, P.; Piekny, A. A Novel Microtubule-Targeting Compound that Disrupts Mitotic Spindle Poles in Human Cells, Scientific Reports. 2022, accepted, manuscript ID: 78e7a22a-32fc-40f0-b189-5da44c1bfb0c.
Hernandez, R.A.; Burchell-Reyes, K.; Passos, A.; Keough Lopez, K.; Forgione, P. Solvent-Free Synthesis of 3,5-Isoxazoles Via 1,3-Dipolar Cycloaddition of Terminal Alkynes and Hydroxyimidoyl Chlorides over Cu/Al2O3 Surface under Ball-Milling Conditions, RSC Advances, 2022, accepted pending minor revisions, manuscript ID: RA-ART-11-2021-008443
Chacón-Huete, F.; Covone, J.; Zaroubi, L.; Forgione, P. Efficient synthesis of bis(5-arylfuran-2-yl)methane scaffolds utilizing biomass-derived starting materials, Eur. J. Org. Chem, 2022, accepted pending minor revisions, manuscript ID: ejoc.202101571.
Liu, J.T.; Jaunky, D.B.; Larocque, K.; Chen, F.; Mckibbon, K.; Sirouspour, M.; Taylor, S.; Shafeii, A.; Campbell, D.; Braga, H.; Piekny, A.; Forgione, P. Design, structure-activity relationship study and biological evaluation of the thieno[3,2-c]isoquinoline scaffold as a potential anti-cancer agent, Bioorg. Med. Chem. Lett. 2021, 52, 128327. DOI (10.1016/j.bmcl.2021.128327)
Messina, C.; Ottenwaelder, X.; Forgione, P. Programmed Synthesis of Tetra-Aryl Thiophenes with Stepwise, Ester-Controlled Regioselectivity, Org. Lett. 2021, 23, 7348. DOI (10.1021/acs.orglett.1c02447)
Chacón-Huete, F.; Lasso, J.D.; Szavay, P.; Covone, J.; Forgione, P. Synthesis of 2,5-Diaryl Nonsymmetric Furans C6-Platform Chemicals via Catalytic Conversion of Biomass and the Formal Synthesis of Dantrolene, J. Org. Chem. 2021, 86, 515. DOI (10.1021/acs.joc.0c02236)
Messina, C.; Douglas, L.Z.; Liu, J.T.; Forgione, P. Successive Pd-Catalyzed Decarboxylative Cross-Couplings for the Modular Synthesis of Non-Symmetric Di-Aryl-Substituted Thiophenes, Eur. J. Org. 2020, 32, 1434. DOI (https://doi.org/10.1002/ejoc.202000780)
Costa Franca, T.C; Costa Bastos, L.; Cuya, T.; Sirouspour, M.; Chacón-Huete, F.; Bendahan, D.; Forgione, P. Microwave-assisted Synthesis and Docking Studies of Phenylureas as Candidates for the Drug Design Against the Biological Warfare Agent Yersinia Pestis, Letters in Drug Design & Discovery, 2020, 17, 633. DOI (https://dx.doi.org/10.2174/1570180816666190710144212)
Liu, J.T.; Hase, H.; Taylor, S.; Salzmann, I.; Forgione, P. Approaching the Interger-Charge Transfer Regime in Molecularly Doped Oligothiophenes by Efficient Decarboxylative Cross-Coupling, Angew. Chem. Int. Ed. 2020, 59, 7146. DOI (https://doiorg.libezproxy.concordia.ca/10.1002/anie.201914458)
Liang, Y.; Manioudakis, J.; Macairan, J-R.; Askari, M.S.; Forgione, P.; Naccache, R. Facile Aqueous-Phase Synthesis of an Ultrasmall Bismuth Nanocatalyst for the Reduction of 4-Nitrophenol, ACS Omega. 2019, 4, 14955. DOI (https://dxdoiorg.libezproxy.concordia.ca/10.1021%2Facsomega.9b01736)
Zhang, X.; Chen, F.; Petrella, A.; Chacón-Huete, F.; Covone, J.; Tsai, T-W.; Yu, C-C.; Forgione, P.; Kwan, D.H. A High-Throughput Glycosyltransferase Inhibition Assay for Identifying Molecules Targeting Fucosylation in Cancer Cell-Surface Modification, ACS Chem. Biol. 2019, 14, 715. DOI (https://dx.doi.org/10.1021/acschembio.8b01123)
Chacón-Huete, F.; Messina, C.; Chen, F.; Cuccia, L.; Ottenwaelder, X.; Forgione, P. Solvent-free mechanochemical oxidation and reduction of biomass-derived 5-hydroxymethyl furfural, Green Chem. 2018, 20, 5261. DOI (https://dx.doi.org/10.1039/c8gc02481b)
Chen, F.; Chacón-Huete, F.; Hassan, E-H.; Forgione, P. Convenient and Inexpensive Route to Sulfonylated Pyridines via SNAr Reaction of Electron-Rich Pyridines by Iron Catalysis, Synthesis. 2018, 50, 1914. DOI (https://dx.doi.org/10.1055/s-0036-1591541)
Rodrigues de Souza, F.; Guimaraes, A.P.; Cuya, R.; Puggina de Freitas, M.; Goncalvez, A.d.S.; Forgione, P.; Franca, T.C.C. Analysis of Coxiella burnetti dihydrofolate reductase via in silico docking with inhibitors and molecular dynamics simulation, J. Biomol. Struct. Dynam. 2017, 34, ahead of print.
Ennis, D.; Despland, E.; Chen, F.; Forgione, P.; Bauce, E. Spruce Budworm Feeding and Oviposition are Stimulated by Monoterpenes in White Spruce Epicuticular Waxes, Insect Sci. 2017, 24, 73t.
Chacón-Huete, F.; Mangel, D.; Ali, M.; Sudano, A.; Forgione, P. High-Value Biomass-Derived 2,5-Furandicarboxylic Acid Derivatives via a Double Decarboxylative Cross-Coupling, ACS Sustainable Chemistry and Engineering 2017, 5, 7071-7076. DOI
Bastos, L.C.; de Souza, F.R.; Guimaraes, A.P.; Sirouspour, M.; Guizado, T.R.C.; Forgione, P.; Ramalho, T.C.; Franca, T.C.C. Virtual screening, docking, and dynamics of potential new inhibitors of dihydrofolate reductase from Yersinia pestis, J. Biomol. Struct. Dynam. 2016, 34, 73t.
Ortgies, D.; Hassanpour, A.; Chen, F.; Woo, S.; Forgione, P. Desulfination as an Emerging Strategy in Palladium-Catalyzed C-C Coupling Reactions, European Journal of Organic Chemistry 2016, 2016, 408-425. DOI
Mangel, D.; Buonomano, C.; Sevigny, S.; Di Censo, G.; Thevendran, G.; Forgione, P. Efficient desulfinative cross-coupling of heteroaromatic sulfinates with aryl triflates in environmentally friendly protic solvents, Heterocycles (Invited paper in honour of Dr. Isao Kuwajima) 2015, 90, 1228-1239. DOI
Chen, F.; Wong, N. W. Y.; Forgione, P. One-Pot Tandem Palladium-Catalyzed Decarboxylative Cross-Coupling and C-H Activation Route to Thienoisoquinolines, Advanced Synthesis and Catalysis 2014, 356, 1725-1730. DOI
Hassanpour, A.; De Carufel, C.A.; Bourgault, S.; Forgione, P. Synthesis of 2,5-Diaryl-Substituted Thiphenes as Helical Mimetics: Towards the Modulation of Islet Amyloid Polypeptide (IAPP) Amyloid Fibril Formation and Cytotoxicity, Chemistry A European Journal 2014, 20, 2522-2528. DOI
Ortgies, D. H.; Forgione, P. A Ligand-Free Palladium Catalyzed Cross-Coupling of Aryl Sulfinates with Aryl Bromides, Synlett 2013. DOI

Sévigny, S.; Forgione, P. Efficient Desulfinylative Cross-Coupling of Thiophene and Furan Sulfinates with Aryl Bromides in Aqueous Media, New J. Chem. 2013, 37, 589. DOI
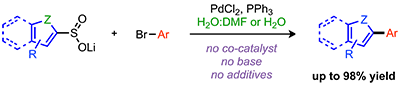
Sévigny, S.; Forgione, P. Palladium-Catalyzed Intermolecular Desulfinylative Cross-Coupling of Heteroaromatic Sulfinates, Chem. Eur. J. 2013, 19, 2256. DOI
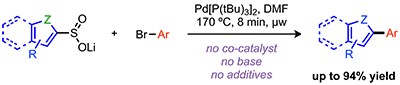
Ortgies, D. H.; Barthelme, A.; Aly, S.; Desharnais, B.; Rioux, S.; Forgione, P. Scope of the Desulfinylative Palladium-Catalyzed Cross-Coupling of Aryl Sulfinates with Aryl Bromides, Synthesis 2013, 45, 694. DOI
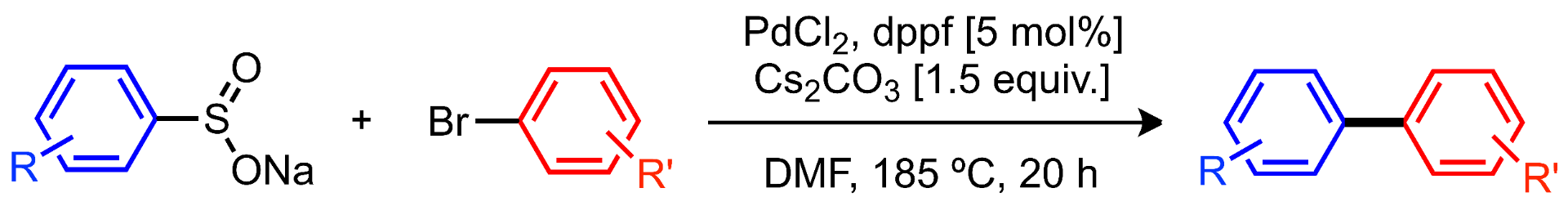
Hassanpour, A.; Sévigny, S.; Forgione, P. Methoxycarbonylsulfamoyl Chloride, Encyclopedia of Reagents for Organic Synthesis [Online], Wiley & Sons, 2012. DOI
Wong, N. W. Y.; Ortgies, D. H.; Forgione, P. 2-(Diphenylphosphino)-2'-(N,N-dimethylamino)-biphenyl, Encyclopedia of Reagents for Organic Synthesis [Online], Wiley & Sons, 2012. DOI
Wong, N.W.Y.; Forgione, P. A One-Pot Double C-H Activation Palladium Catalyzed Route to a Unique Class of Highly Functionalized Thienoisoquinolines, Org. Lett. 2012, 14, 2738. DOI

Bilodeau, F.; Brochu, M.-C.; Guimond, N.; Thesen, K.H.; Forgione, P. Palladium-Catalyzed Decarboxylative Cross-Coupling Reaction Between Heteroaromatic Carboxylic Acids and Aryl Halides, J. Org. Chem. 2010, 75, 1550. DOI

Llinas-Brunet, M.; Bailey, M.D.; Goudreau, N.; Bhardwaj, P.K.; Bordeleau, J.; Bos, M.; Bousquet, Y.; Cordingley, M. G.; Duan, J.; Forgione, P.; Garneau, M.; Ghiro, E.; Gorys, V.; Goulet, S.; Halmos, T.; Kawai, S.H.; Naud, J.; Poupart, M.-A.; White, P.W. Potent and Selective Noncovalent Linear Inhibitor of the Hepatitis C Virus NS3 Protease (BI 201335), J. Med. Chem.2010, 53, 6466. DOI
Braun, A.; Cho, I.H.; Ciblat, S.; Clyne, D.; Forgione, P.; Hart, A.C.; Huang, G.; Kim, J.; Modolo, I.; Paquette, L.A.; Peng, X.; Pichlmair, S.; Stewart, C.A.; Wang, J.; Zuev, D. Enantioselective Synthesis of Structurally Intricate and Complementary Poly-Oxygenated Building Blocks of Spongistatin 1 (Altohyrtin A), Coll. Czech. Chem. Comm. 2009, 74, 651. DOI
James, C.A.; Coelho, A.L.; Gevaert, M.; Forgione, P.; Snieckus, V. Combined Directed ortho and Remote Metalation-Suzuki Cross-Coupling Strategies. Efficient Synthesis of Heteroaryl-Fused Benzopyranones from Biaryl O-Carbamates, J. Org. Chem. 2009, 74, 4094. DOI
Naud, J.; Lemke, C.; Goudreau, N.; Beaulieu, E.; White, P.D.; Llinas-Brunet, M.; Forgione, P. Potent Triazolyl-Proline-Based Inhibitors of HCVNS3 Protease, Bioorg. Med. Chem. Lett. 2008, 18, 3400. DOI
Kawai, S.H.; Bailey, M.D.; Halmos, T.; Forgione, P.; LaPlante, S.R.; Llinas-Brunet, M.; Naud, J.; Goudreau, N. The Use of Chemical Double-Mutant Cycles in Biomolecular Recognition Studies: Application to HCV NS3 Protease Inhibitors, Chem. Med. Chem. 2008, 3, 1654.
Ciblat, S.; Kim, J.; Stewart, C.A.; Wang, J.; Forgione, P.; Clyne, D.; Paquette, L.A. A Modular Approach to Marine Macrolide Construction. 4. Assembly of C36-C51 and C29-C44 Building Blocks and Evaluation of Key Coupling Reactions Targeting Spongistatin 1 (Altohyrtin A), Org. Lett. 2007, 9, 719. DOI
Forgione, P.; Fader, L. D. Product class 3: propargylic alcohols, Science of Synthesis, ed. Prof. J. Clayden, 2007.
Forgione, P.; Brochu, M.-C.; St-Onge, M.; Thesen, K.H.; Bailey, M.D.; Bilodeau, F. Unexpected Intermolecular Pd-Catalyzed Cross-Coupling Reaction Employing Heteroaromatic Carboxylic Acids as Coupling Partners, J. Am. Chem. Soc. 2006, 128, 11350. DOI
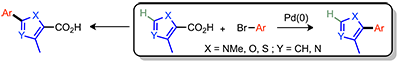
Fort, Y.; Forgione, P. (first update) 2-Fluoropyridine, Electronic Encyclopedia or Reagents for Organic Synthesis, ed. L.A. Paquette, 2007. DOI
Forgione, P. 3-Fluoroquinoline, Electronic Encyclopedia or Reagents for Organic Synthesis, ed. L.A. Paquette, 2006. DOI
Bhardwaj, P.K.; Forgione, P. 2,6-Difluoropyridine, Electronic Encyclopedia or Reagents for Organic Synthesis, ed. L.A. Paquette, 2006. DOI
Bhardwaj, P.K.; Forgione, P. 3-Fluoropyridine, Electronic Encyclopedia or Reagents for Organic Synthesis, ed. L.A. Paquette, 2006. DOI
Laurent, A.; Villalva-Servin, N.P.; Forgione, P.; Wilson, P.D.; Smil, D.V.; Fallis, A.G. Part 1: Efficient Strategies for the Construction of Variably Substituted Bicyclo[5.3.1]Undecenones (AB Taxane Ring Systems), Can. J. Chem. 2004, 82, 215. DOI
Forgione, P.; Fallis, A.G. Metal Mediated Carbometallation of Alkynes and Alkenes Containing Adjacent Heteroatoms, Tetrahedron 2001, 57, 5899. DOI
Melekhov, A.; Forgione, P.; Legoupy, S.; Fallis, A.G. Tether-Contolled Cycloadditions for the Asymmetric Synthesis of Decalins, Org. Lett. 2000, 2, 2793. DOI
Forgione, P.; Wilson, P.D.; Yap, G.A.; Fallis, A.G. A Carbometallation Intramolecular Cycloaddition Strategy for the AB-Taxane Ring and an Enone Accelerated Cope Rearrangement to Bicyclo[2.2.2]octanones, Synthesis 2000, 921. DOI
Forgione, P.; Fallis, A.G. Magnesium Mediated Carbometallation of Propargyl Alcohols: Direct Route to Furans and Furanones, Tetrahedron Lett. 2000, 41, 17. DOI
Forgione, P.; Fallis, A.G. Magnesium Mediated Carbometallation of Propargyl Alcohols: Direct Route to Dihydrodienes and Enediyne Alcohols, Tetrahedron Lett. 2000, 41, 11. DOI
Fallis, A.G.; Forgione, P.; Woo, S.; Py, S.; Harwig, C.; Rietveld, T. Organometallic Reagents and Protocols for Synthesis, Polyhedron 2000, 19, 533. DOI
Saban, M.; Liebermann, G.; Jay, A.; Shi, A.C.; Ro, N.; Dale, W.; Forgione, P. Piloting of Anionic Polymerization of Styrene in Tetrahydrofuran, Can. J. Chem. Eng. 2000, 78, 320. DOI

