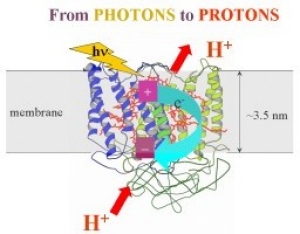
Our group uses an interdisciplinary approach that stems from concepts in physics, chemistry and biology to study photosynthetic processes at the molecular level. We specialize in:
- Monitoring light-induced electron and proton transfer reactions in photosynthesis,
- Study of lipid-protein interactions and
- Artificial membrane characterization.


 Group members 2016 (l-r) Chuck Protheroe, Dr. Laszlo Kalman and Sasmit Deshmukh
Group members 2016 (l-r) Chuck Protheroe, Dr. Laszlo Kalman and Sasmit Deshmukh

 Perkin-Elmer MP44 spectrofluorimeter interfaced with a LeCroy WaveSurfer 422 digital storage oscilloscope. The web-enabled interface program was written by the NanoScience Group facility manager Dr. Rolf Schmidt.
Perkin-Elmer MP44 spectrofluorimeter interfaced with a LeCroy WaveSurfer 422 digital storage oscilloscope. The web-enabled interface program was written by the NanoScience Group facility manager Dr. Rolf Schmidt.
 Transient absorption spectrophotometer with capabilities to measure time resolved absorption changes from 30 ns to 10 s between 330-900 nm.
Transient absorption spectrophotometer with capabilities to measure time resolved absorption changes from 30 ns to 10 s between 330-900 nm.
 Spectrophotometer with a large wavelength range (175-3300 nm) and ultra low photometric noise levels (10‑5 absorption units). The temperature can be controlled from 0-100 °C with a Peltier element.
Spectrophotometer with a large wavelength range (175-3300 nm) and ultra low photometric noise levels (10‑5 absorption units). The temperature can be controlled from 0-100 °C with a Peltier element.
 Dual Polarization Interferometry (DPI) is an emerging analytical biophysical technique that allows changes in the structure to be followed and quantified in real time. The applied method simultaneously resolves the changes in thickness and surface density in atomic resolution by probing the structure in one dimension using non-diffractive optics.
Dual Polarization Interferometry (DPI) is an emerging analytical biophysical technique that allows changes in the structure to be followed and quantified in real time. The applied method simultaneously resolves the changes in thickness and surface density in atomic resolution by probing the structure in one dimension using non-diffractive optics.